Health research needs informed and trustful research participants, samples, data, secure infrastructures and databases, etc. And it also needs clear rules that could be easily understood by researchers.
Among the rules that European researchers have to follow there is the EU General Data Protection Regulation (GDPR). However, it is complex to interpret for researchers: provisions related to research are fuzzy and vague, leaving space for National States’ different interpretation and thus making harmonization difficult to achieve.
The GDPR encourages codes of conduct under art. 40 and 41 to ‘translate’ into practice the GDPR provisions and simplify its implementation. Indeed, a code of conduct could:
- contribute to the proper application of the GDPR, taking into account the specific features of processing personal data in the area of health;
- clarify and specify certain rules of the GDPR for controllers who process personal data for purposes of scientific research in the area of health;
- help demonstrate compliance by controllers and processors with the regulation;
- help foster transparency and trust in the use of personal data in the area of health research
Our Initiative
The initiative for drafting a Code of Conduct for Health Research was launched in 2015, pushed by scientific and legal experts of BBMRI-ERIC, the research infrastructure for biobanks and biomolecular resources. By 2017, BBMRI-ERIC began to exchange with policy makers, fellow research infrastructures as well as other stakeholders from academia and industry concerned with human research data and patient advocacy groups, such as EFPIA, ESR, ECRIN, EURORDIS, or CESSDA, and around 2018 the drafting action effectively started.
At the beginning of 2020, many points were already discussed and drafted, but the Covid-19 emergency needs took precedence. However, the pandemic experience provided some interesting case studies for the Code and has pushed the willingness of stakeholders to contribute on the top of the list.
After the pause caused from the pandemic, the drafting of the Code restarted and its completion became a priority for BBMRI-ERIC. Since then, the experts have been meeting regularly to draft the rules of the Code.
The Code of Conduct generates more and more interest each year. E.g.: By 2024, more than 160 individuals representing around 90 organizations indicated their interest and support for the Code.
In 2024, a Code of Conduct under the GDPR is more relevant and more pressing than ever. It remains a challenging task and requires a solid legal and meaningful practical framework for the life sciences research.
Our Timeline
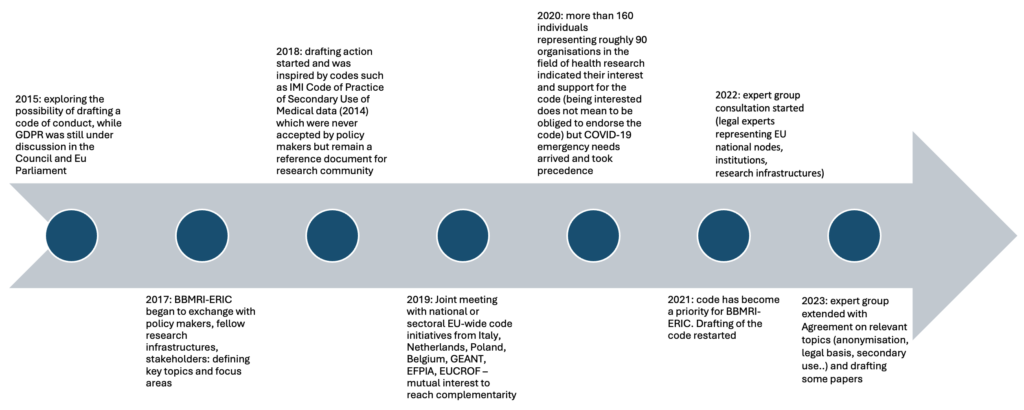
The Development Process
The development process has been planned in waves:

View our summary presentation
You can access a PDF of the presentation about the Code of Conduct here.
Contact us!